CURRENT RESEARCH INTERESTS

Nuclear Factor kappa B (NF-κB)
My work in this area is almost all in collaboration with Mike White (Manchester). Most of our work Is concerned with understanding the function of the oscillations in the NF-κB system, whereby the transcription factor NF-κB locates in and out of the nucleus in a periodic fashion when the system is activated. These oscillations were discovered in Mike's lab, initially in cell cultures but now in primary cells as well. My basic hypothesis in this area is that NF-κB acts as an information hub with the oscillations allowing it to carry much more information than would be possible otherwise.
Discovered over 25 years ago,the NF-κB transcription factor controls inflammation and in different contexts has varying effects on cell death and cell division. It is found in almost all animal cell types and is involved in cellular responses to stimuli such as stress, cytokines, free radicals, ultraviolet irradiation, oxidized LDL, and bacterial or viral antigens. It plays a key role in regulating the immune response to infection. Incorrect regulation of NF-κB has been linked to cancer, inflammatory, and autoimmune diseases, septic shock, viral infection, and improper immune development. NF-κB has also been implicated in processes of synaptic plasticity and memory.
NF-κB binding sites are found in the promoter regions of around 300 genes, including cytokines (e.g. TNFα, LTβ, IL-1 and GM-CSF). NF-κB is activated by various stress stimuli, including inflammatory cytokines such as TNFα and IL-1β. NF-κB signalling involves phosphorylation of IκB proteins by the IKK kinase complex. This allows the translocation of an NF-κB dimer (typically RelA:p50) into the nucleus.
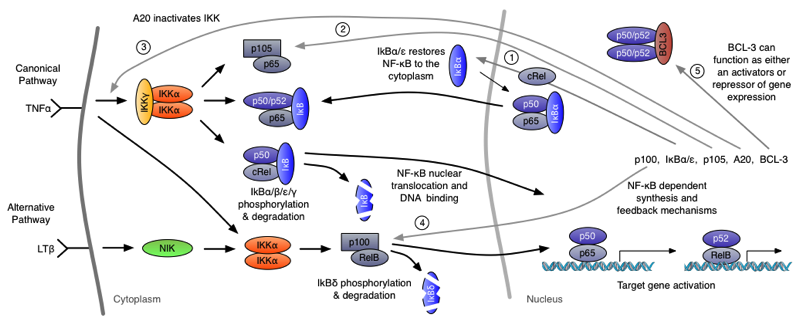
A schematic representation of the NF-κB network.
Real-time fluorescence imaging and mathematical modelling have shown that the activity of the NF-κB system can be oscillatory. After stimulation with TNFa, target gene expression can be regulated by negative feedback loops that modulate the cytoplasmic-nuclear translocation of NF-κB. One of these feedbacks is mediated by IkBa, which upon binding to NF-kB in the nucleus shuttles the NF-κB protein complex back to the cytoplasm. These oscillations have been observed in single cells expressing the fluorescently labeled NF-κB subunit RelA and IkBa in cell lines and primary cells.
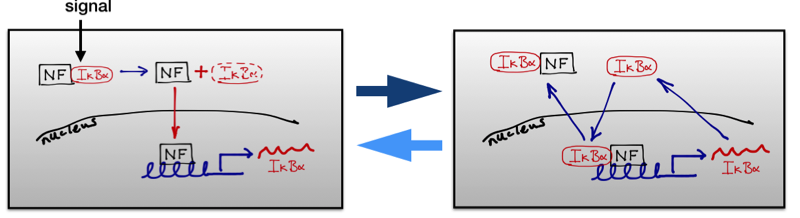
A schematic representation of how the NF-κB network oscillations work. The incoming signal causes phosphorylated of the inhibitory IκBα which is bound to the NF-κB, allowing the transcription factor NF-κB to be liberated and to enter the nucleus. There it turns on the transcription of the IκBα gene causing the production of IκBα mRNA which is then translated in the cytoplasm into protein. This returns to the nucleus, binds to the NF-κB and pulls it back into the cytoplasm so the whole process can run again.
Papers
To address the sources of variability relevant to single-cell data, namely, intrinsic noise due to the stochastic nature of reactions, and extrinsic noise arising from the cell-to-cell variation of kinetic parameters we derive a dynamic state space model for molecular populations, extend it to a hierarchical model and apply it to multiple single-cell time series data.
Quantifying intrinsic and extrinsic noise in gene transcription using the linear noise approximation: an application to single cell data.
Bärbel Finkenstädt, Dan J. Woodcock, Michal Komorowski, Claire V.Harper, Julian R.E. Davis, Mike R.H. White, David A. Rand. Annals of Applied Statistics , (2013) 7 (4) 1960–1982.
An algorithm that can estimate the transcription rates of genes even when transient transfections with variable gene copy numbers are involved. This can be used, for example, in projects where it is necessary to work with many different constructs.
A hierarchical model of transcriptional dynamics allows robust estimation of transcription rates in populations of single cells with variable gene copy number.
Dan J. Woodcock, Keith W. Vance, Michał Komorowski, Georgy Koentges, Bärbel Finkenstädt and David A. Rand. Bioinformatics (2013), pages 1–7 doi:10.1093/bioinformatics/btt201
The basic mathematical tools you need for experimental design and sensitivity analysis for stochastic regulatory or signalling systems. Uses the linear noise approximation.
Sensitivity of stochastic chemical kinetics models.
M. Komorowski, M. Costa, D. A. Rand, and M. L. Stumpf, PNAS 2011 108 (21) 8645-86
Transcription dynamics from two loci in real time in single cells. Evidence for a refractory period in the inactivation phase of transcription. New theoretical techniques for reconstructing transcription from imaging data.
Dynamic Analysis of Stochastic Transcription Cycles.
C. V. Harper, B. Finkenstädt, D. Woodcock, S Friedrichsen, S. Semprini, L Ashall, D. Spiller, J. J. Mullins, D. A. Rand, J. R.E. Davis, M. R. H. White. PLoS Biology 9(4): e1000607. doi:10.1371/journal.pbio.1000607
Multiparameter experimental and computational methods that integrate quantitative measurement and mathematical simulation of these noisy and complex processes are required to understand the highly dynamic mechanisms that control cell plasticity and fate.
Measurement of Single Cell Dynamics.
D G Spiller, C. D. Wood, D. A. Rand, M. R. H. White. Nature 465 (2010) 736-745
Describing the heterogenious response of low-dose stimulation of the NF-kB system
Physiological levels of TNFa stimulation induce stochastic dynamics of NF-kB responses in single living cells.
D. A. Turner, P. Paszek, D. J. Woodcock, D. E. Nelson, C. A. Horton, Y. Wang, D. G. Spiller, D. A. Rand, M. R. H. White, and C. V. Harper, Journal of Cell Science 123: 2834-2843 (2010)
Feedbacks of NF-kappaB optimised to increase single-cell heterogeneity and population robustness.
Population Robustness Arising From Cellular Heterogeneity.
P. Paszek, S. Ryan, L. Ashall, K. Sillitoe, C. V. Harper, D. G. Spiller, D. A. Rand and M. R. H. White, PNAS doi/10.1073/pnas.0913798107
Stimulation frequency modulates differential gene expression by NF-kappaB; IkappaBepsilon feedback regulates heterogeneithy of oscillations; and the structure and role of A20 feedback is predicted.
Pulsatile stimulation determines timing and specificity of NF-kappa B-dependent transcription.
L. Ashall, C.A. Horton, D.E. Nelson, P. Paszek, C.V. Harper, K. Sillitoe, S. Ryan, D.G. Spiller, J.F. Unitt, D.S. Broomhead, D.B. Kell, D.A. Rand, V. Sée, and M.R.H. White. Science 324 (2009) 242-246
New summation theorems that substantially generalise previous results to dynamic non-stationary solutions such as periodic orbits and transient signals and apply to both autonomous and non-autonomous systems such as forced nonlinear oscillators.
Network control analysis for time-dependent dynamical states.
D. A. Rand. Dynamics and Games in Science, in honour of Mauricio Peixoto and David Rand. Springer 2010.
Under construction